The Houston innovation ecosystem has been bursting at the seams with news from innovative tech companies and disruptive Houston startups as we fly through the final quarter of 2021.
In this roundup of short stories within Houston innovation, a Texas energy tech company gets selected for a prestigious program, a med device company heads to clinical trials, a startup launches a crowdfunding campaign, and more.
The Postage launches crowdfunding campaign
![]()
The Postage is looking for financial support with its new campaign. Photo courtesy of The Postage
The Postage, a Houston-based, full-service digital platform to help organize affairs to make after-life planning a smoother process for families, has announced the launch of a crowdfunding campaign through MicroVentures.
"This crowdfunding offering is selling crowd notes to raise maximum offering proceeds of $500,000 with a minimum investment of $100," according to a news release. "We currently anticipate closing this offering on April 4, 2022."
More information on this offering can be found at: https://invest.microventures.com/offerings/the-postage.
Emily Cisek co-founded the company after she experienced an overwhelming experience following a death in her family.
"I just knew there had to be a better way, and that's why I started The Postage," Cisek says on an episode of the Houston Innovators Podcast. "My background had historically been in bringing offline businesses online, and I started doing some research on how I could make this space better. At the time, there really wasn't anything out there."
Texas company selected for Chevron Technology Ventures Catalyst Program
![]()
This Texas company has joined CTV's startup program. Photo via Getty Images
SeebeckCell Technologies, while based in Arlington, Texas, is no stranger to the Houston innovation ecosystem. The startup was in the first class of the Rice Alliance Clean Energy Accelerator, participated in the MassChallenge Texas Houston cohort, and is a member at Greentown Houston. The company announced earlier this month a new Houston association as it was invited to participate in the Chevron Technology Ventures Catalyst Program to develop further their technology platform designed to recover industrial waste heat energy, increasing energy consumption efficiency, and eliminating battery replacement in IoT applications, according to a news release.
"SeebeckCell is excited to be supported by Chevron, a technology leader in the energy market," says Ali Farzbod, co-founder and CEO of Seebeckcell Technologies, in the release. "This is inspiring hope in the scientific community as we see Chevron continue to back commercializing academically developed technologies that provide potential solutions for addressing climate change. Through collaboration and partnership, we're able to grow our startup and we're grateful for participating in the Rice Alliance Clean Energy Accelerator that helped connect us with Chevron."
SeebeckCell Technologies is helping petroleum and gas industries and emerging markets solve energy waste with an innovative liquid based thermoelectric generator.
VenoStent heads to clinical trials
![]() VenoStent
VenoStentVenoStent has reached the clinical trials stage. Photo via venostent.com
VenoStent Inc. has announced successful enrollment in its initial feasibility clinical trial. The med device startup is a tissue engineering company that's developing smart polymer wraps to transform the efficacy of the vascular surgery industry, which sees five million operations each year.
"We are very pleased to announce that we have successfully enrolled twenty end-stage renal disease patients in our initial feasibility study taking place in Asuncion, Paraguay," says Tim Boire, CEO., in a news release "After years of development, we are confident that our bioabsorbable wrap technology can have a positive impact on the lives of patients that require hemodialysis to sustain life. This is a major milestone toward our mission to improve the quality and length of life for end-stage renal disease patients, as well as others needing vascular surgery."
VenoStent is an alum of TMC Innovation's accelerator and has been named a most promising company by Rice Alliance.
Cart.com announces latest partnership
![]()
Cart.com has a new partner, which has increased access to tools for its clients. Photo via cart.com
Houston-based Cart.com, an end-to-end ecommerce services provider and Amazon competitor, has announced yet another new partnership. The company has teamed up with Extend, which provides modern extended warranties and product protection plans. The partnership means that Cart.com merchants have access to a new revenue stream and new ways to increase customer satisfaction by leveraging Extend's platform and technology-enabled proprietary insurance stack.
"Like Cart.com, Extend is fixing the fractured ecommerce ecosystem by providing a truly innovative, effortless, and easy-to-understand service for both merchants and their customers," says Omair Tariq, Cart.com co-founder and CEO, in a news release. "By creating seamless solutions to serve brands, we empower them to focus completely on their customers. The partnership with Extend fits squarely in this view; anyone who has wrangled with extended warranty claims in the past understands the friction involved. Extend is rewriting the rules for product protection and customer service while Cart.com takes care of everything from the factory floor to the customer door. Through this partnership with Extend, we're now seamlessly covering the post-purchase experience too."
Extend launched in 2019 — a time when only the top 1 percent of merchants could offer extended warranties and protection plans to help their customers, according to the release. Now, Extend is valued at $1.6 billion, has raised over $315 million in venture capital, is on track to sell more than three million protection plans in 2021.
"The relationship between an ecommerce company and its customer doesn't end with the sale," says Woodrow Levin, co-founder and CEO of Extend, in the release. "Our technology will allow Cart.com's clients to continue to engage customers after they make a purchase, unlocking opportunities to increase brand loyalty, open new revenue channels, and create lasting customer relationships. Together, we're empowering clients to deliver a better experience for customers and we are excited to continue to build on that vision."
Campus Concierge rebrands to Clutch with revamped website

A Houston startup has just flipped a switch. Image via thatsclutch.com
Campus Concierge is now Clutch, the Houston-based startup announced on its Facebook page last month. The new name also came with a revamped website.
Madison Long and Simone May had the idea for the company when they were undergraduate students at Purdue University and their only option for scoping out basic services — like getting their hair done or hiring a DJ for an event or a photographer for graduation photos — was to ask around among older students. Launched earlier this year, the platform is a marketplace to connect students who have skills or services with potential clients in a safe way. The company, which was a member of DivInc's inaugural Houston accelerator, launched on three college campuses this year — Texas Southern University, Rice University, and Prairie View A&M.
"Building community is so critical given the fact that it's nerve-wracking any time to ask someone for help — especially now that you are coming back to school after a year of being virtual," Long, CEO and co-founder of Clutch, previously told InnovationMap.






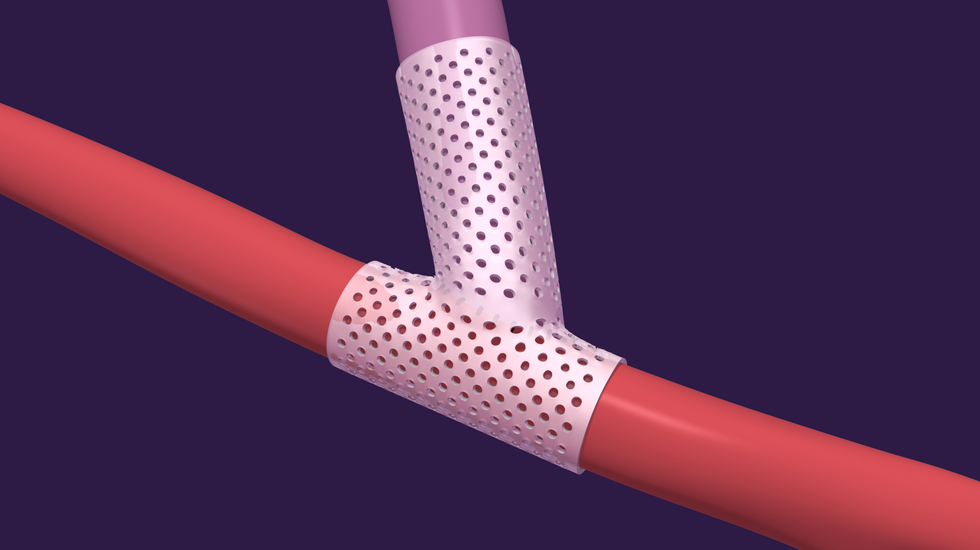 VenoStent's novel therapeutic medical device is a bioabsorbable wrap. Image courtesy of VenoStent
VenoStent's novel therapeutic medical device is a bioabsorbable wrap. Image courtesy of VenoStent Tim Boire is the CEO of VenoStent. Photo via LinkedIn
Tim Boire is the CEO of VenoStent. Photo via LinkedIn

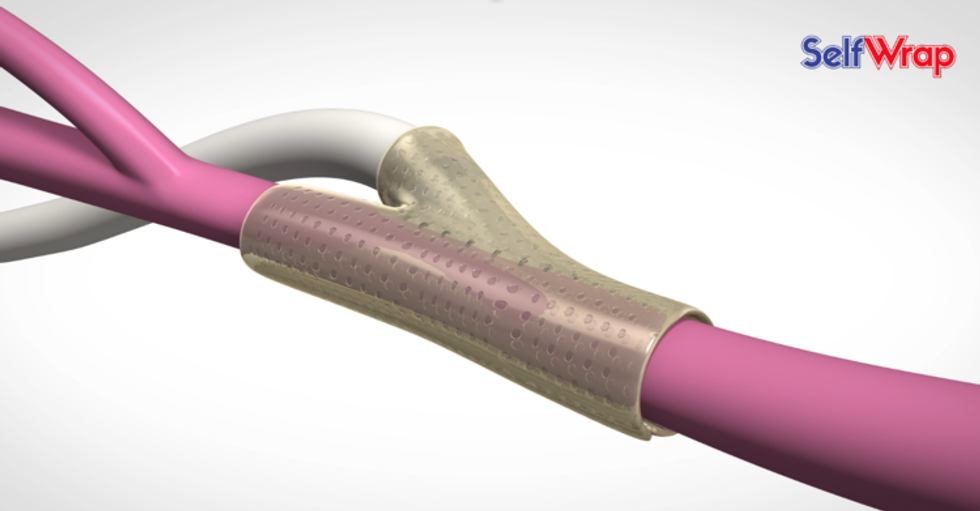 VenoStent
VenoStent