Houston innovators had no need to beware the ides of March this year. With all the excitement from SXSW, CERAWeek, and Houston Tech Rodeo this month, there might be some headlines you may have missed.
In this roundup of short stories within Houston startups and tech, Houston startups announce new funding and partnerships, while a Texas VC raises its largest fund yet.
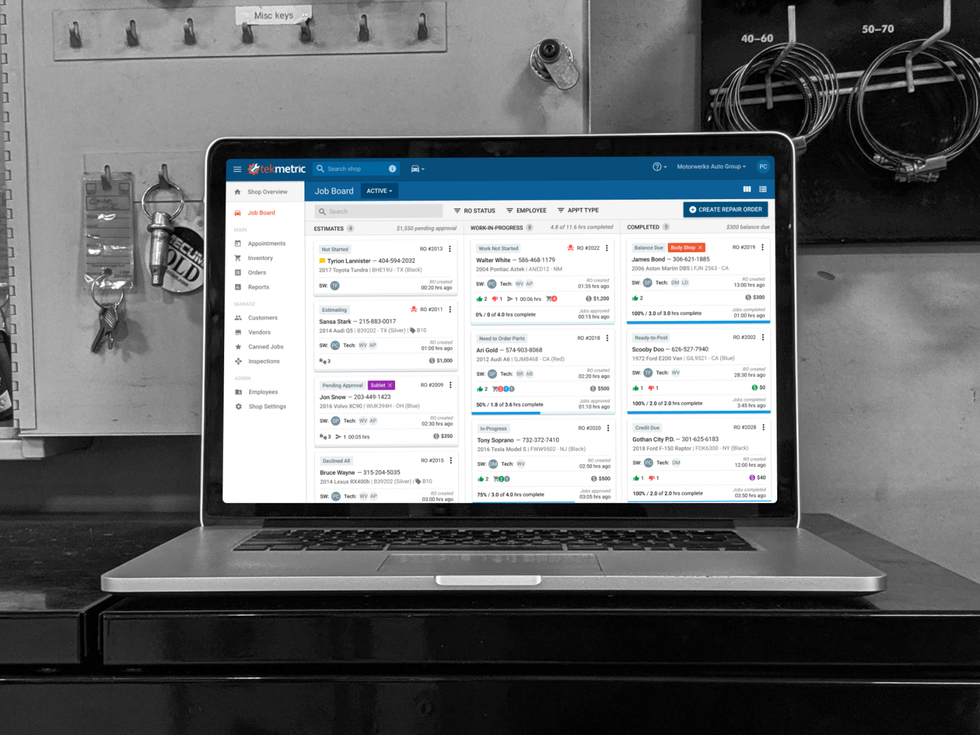
Tekmetric closes recent fundraising round

A Houston software company has raised an undisclosed amount of funding. Photo via tekmetric.com
Tekmetric, a cloud-based shop management system for automotive repair shops, announced the close of its growth investment from California-based Susquehanna Growth Equity. The details of the round were not disclosed, but, according to a news release, the fresh funds will go toward growing Tekmetric's engineering and technical teams and expansion across the United States.
Launched three years ago by Prasanth Chilukuri and Sunil Patel, co-founders and co-CEOs, Tekmetric's SaaS solution provides shop owners with digital inspections, integrated payments, and more of their business needs.
“Since our launch in 2016, Tekmetric has always aspired to deliver the greatest possible value to auto repair shop owners who partner with us to run their business,” says Chilukuri in the release. “Susquehanna’s deep industry expertise and support of product-led growth makes the company an ideal partner as we scale our business, boosting our platform’s advanced products and providing the highest caliber of service for our customers.”
The platform provides both convenience and security for its users.
“As a former shop owner myself, I know how difficult it can be to find a system like Tekmetric that shop owners can trust with their business,” says Patel. “At Tekmetric, we strive to build strong relationships with our users to support their business growth. The SGE team has the same mindset, which makes them an ideal partner as Tekmetric continues to grow in the industry.”
The Postage taps new financial planning partner

The Postage has a new strategic partner. Photo courtesy of The Postage
Houston-based legacy and estate planning software platform
The Postage has announced a new partnership with Austin-based Whitwell & Co., LLC, an investment management and financial planning firm.
The Postage platform, which will now be available for Whitwell's clients with the new collaboration, range from important information and documents management, estate planning document creation, end-of-life planning, and memory and message storing.
“Whitwell & Co. focuses on supporting their clients through the myriad of choices that arise during planned and unplanned life events and transitions. The Postage fits right into that, and we are thrilled for the opportunity to share our platform with their clients in their planning and organization efforts,” says Emily Cisek, CEO and co-founder of The Postage. “Our hope is to grow awareness of the streamlined digital solutions available and provide Whitwell’s clients the opportunity to create estate planning documents, easily store and safeguard critical information that families will need access at all phases of life. We look forward to providing clients of Whitwell & Co. a comprehensive planning and preparation service that delivers peace of mind to their families.”
The B2B partnership takes effect this month. The Postage, which was founded in 2019, is also closing its crowdfunding campaign on April 4.
“As a company, we are built upon the principles of an innovative approach to investment management and financial planning,” says Stefan Whitwell, CEO at Whitwell & Co. “This partnership is an important approach for us to offer our clients as we progress into the digital age. Having been around families who have had to experience the loss of a loved one, I see the need for a service like The Postage. Too often many are unsure of next steps, where documentation lives, and even last wishes.”
Austin venture capital firm with Houston portfolio companies raised $250M fund

S3 Ventures has fresh funding and eyes for Texas startups. Photo via S3
Billed as the "largest venture capital fund focused on the state of Texas," S3 Ventures's recently announced $250 million Fund VII is focused on investing in Texas startups.
S3 Ventures usually invests $500,000 to $10 million in seed, series A or series B rounds with the capacity to invest more than $20 million throughout the life of a company. The firm has made more than 50 investments since it was founded in 2005 and has more than 25 active portfolio companies and over 20 exits.
“In our first 17 years, we have been fortunate to partner with truly visionary founders who have transformed the way we work, live and heal,” says S3 Managing Director Brian R. Smith in a news release. “We look forward to working with many more in the years ahead.”
The firm has Houston startups in its portfolio: BrainCheck, a provider of interactive cognitive assessment and care planning technology; Saranas, an early bleed detection system; and BuildForce, a construction labor marketplace.
“We believe that by 2030, Texas could be the second-largest technology ecosystem in the country,” Smith said. “That growth is being driven by long-term demographic shifts and broad-based economic strength of not just Austin, but also Dallas, Houston and San Antonio.”
Saranas announces new patent

This Houston medical device company has reached another step in commercialization. Photo courtesy of Saranas
Houston-based early bleed detection medical device company Saranas has been granted a new patent from the Department of Commerce’s United States Patent and Trademark Office. The patent, titled “Access Closure with Bleed Monitoring,” allows for embedding a vascular access closure device with the company’s proprietary bleed monitoring technology.
“As we continue to grow our commercial presence with the Early Bird, we are pleased to secure this important patent that is designed to further expand the implementation of our differentiated bleed monitoring technology,” says Saranas CEO James Reinstein in a news release. “This patent award demonstrates Saranas’ commitment to innovation and further strengthens our intellectual property portfolio.”
At the end of 2021, Saranas announced its first patient in its clinical trials at Morristown Medical Center in Morristown, New Jersey. The trial will eventually enroll up to 265 patients across the U.S.
"We have been using the Early Bird in our clinical practice for the past two years, and the current design of incorporating a fully functional introducer sheath with bleed detection allows for seamless integration into high risk interventional cardiovascular procedures," Dr. Philippe Généreux, interventional cardiologist, says. "Embedding bleed detection directly onto a vascular closure device is the eventual next step and has the potential to become the standard of care across all types of vascular access procedures.”
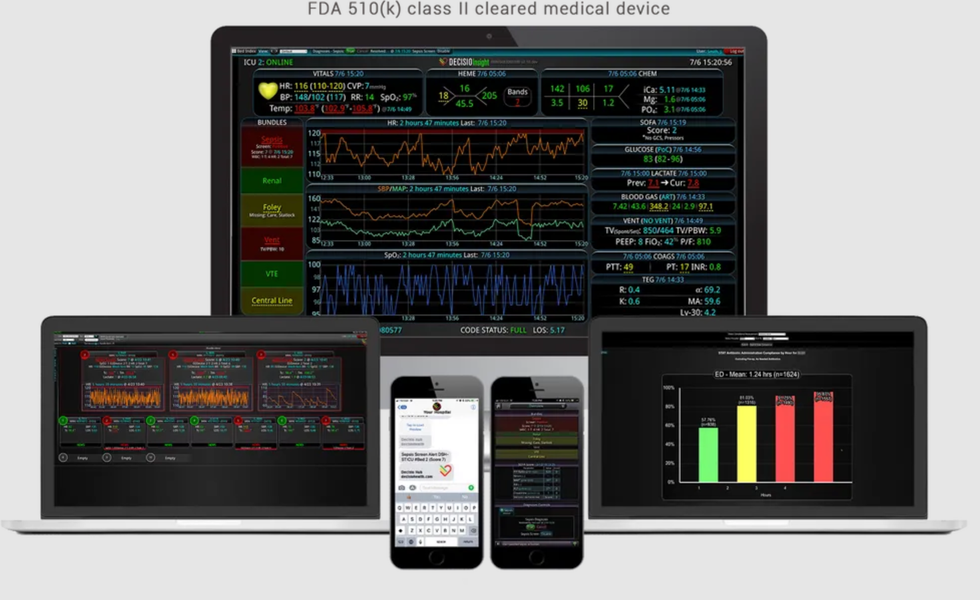
DECISIO announces new product

DECISIO has a new product on the market. Photo via decisiohealth.com
Houston-based DECISIO has created a suite of customizable clinical decision support tools has announced a new product: EnvisionIQ. The new tool provides templated real-time and customized compliance reports to improve operational efficiency.
EnvisionIQ is a hospital's real-time data and visualization solution enables health systems to benchmark their clinicians, units, and hospitals to accelerate improvements, reduce variation, and expedite data collection for agency reporting requirements.
"Clinical benchmarking tools are essential to enable health systems to quickly identify improvement opportunities that have substantial impact. The addition of EnvisionIQ to our product portfolio allows DECISIO to provide comprehensive surveillance and analytics platforms to benefit hospitals in many capacities," says Paul Sinclair, chief revenue officer at DECISIO, in a news release.
Customers can tap into DECISIO's new product with or without integration with its flagship product, InsightIQTM, which was launched in 2015. The company raised a $13 million series B round in 2019.