California femtech leader integrates Houston-founded conversational AI tool
girl gang
Willow users, meet Ema — you're new best AI-enabled friend.
Houston-founded Ema (née SocialMama), an AI resource for maternal health support, has been loaded onto the Willow Innovations Inc. app, a platform for breastfeeding mothers.
"We are thrilled to integrate our conversational AI platform into the Willow App and leverage the power of advanced technology to meet women where they are with the information they need," Amanda Ducach, founder of Ema, says in a news release. "Ema and Willow have a shared mission to make lasting improvements in women's health and we built a one-of-a-kind solution that addresses the unique challenges mothers face regularly.
"Our partnership represents a critical advancement for today's mothers and demonstrates how responsible AI can improve the maternal care experience," she continues.
Now, users on the app can utilize Ema's HIPPA-secure tool that pulls from a comprehensive, proprietary database of expert-backed information. The conversational AI chatbot responds prioritizing speed, accuracy, and empathy and compassion for users. Ema reports that their users "will feel like they are talking with their favorite postpartum (labor and delivery) nurse."
"New moms don't have enough support — for their feeding and parenting journey, or in their postpartum care. We're thrilled to partner with Ema to offer moms personalized information along this journey," adds Sarah O'Leary, CEO of Willow. "AI tools, when implemented thoughtfully, can help close gaps in delivering personalized guidance at an efficient scale and I'm excited about the impact Ema can have on the well-being of mothers in our Willow community."
Founded in 2018 as a way to connect new moms, Ema evolved with a major rebrand and pivot to AI-backed tools last year.
Willow was launched in 2014 and released the first wearable, in-bra breast pump in 2017.
- Houston AI femtech startup raises $2M round to expand platform to B2B opportunities ›
- Houston energy data software platform expands into Europe, hires new execs ›
- Houston startup designing emergency response drones lands $5.2M in seed funding ›
- Exclusive: Houston startup rebrands to provide AI chat tool focused on women’s health ›

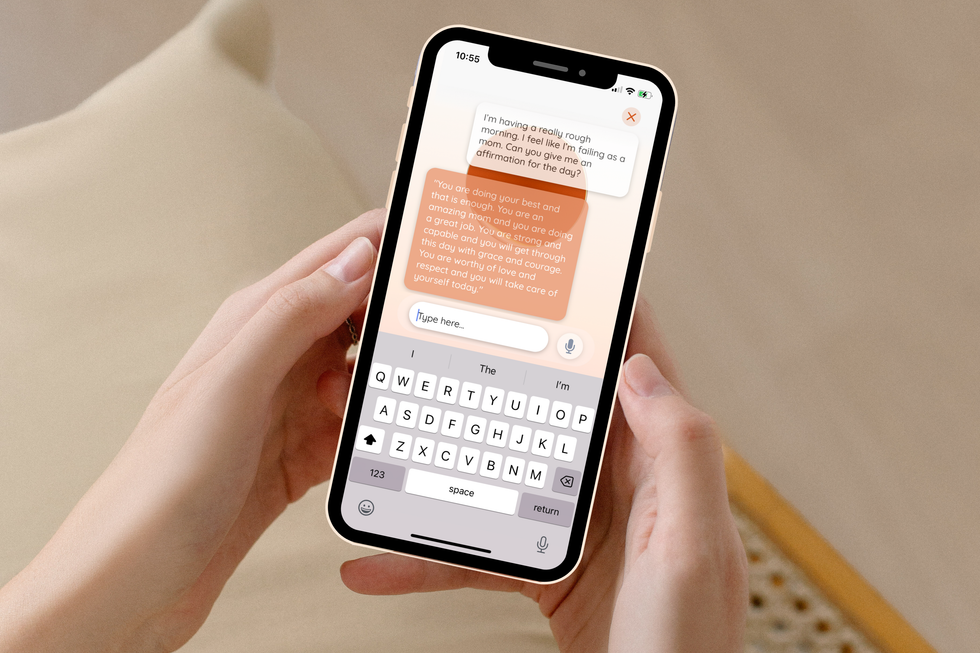 The new platform, deemed ema, operates as an AI-based chat for women to engage with. Screenshot courtesy of ema
The new platform, deemed ema, operates as an AI-based chat for women to engage with. Screenshot courtesy of ema Amanda Ducach founded the company in 2019. Photo via Twitter
Amanda Ducach founded the company in 2019. Photo via Twitter