Houston unicorn raises $200M series D, startup snags national award, and more innovation news
Short stories
It's been a busy month so far with plenty of Houston startup news, major ecosystem events, and more — and there might be some headlines you may have missed.
In this roundup of short stories within Houston startups and tech, Solugen raises another mega round of funding, CorInnova snags a prestigious award, applications are open for two programs, and more.
Houston unicorn chemicals company raises $200M series D

Solugen closed its Series d funding round at $200 million. Photo via Getty Images
Houston-based Solugen has announced its latest round of investment to the tune of $200 million. The company, which reached unicorn status after its $357 million series C round last year, uses its patented Bioforge processes to produce "green" chemicals from bio-based feedstocks.
"Solugen is reimagining the chemistry of everyday life with enzymes found in nature. We make chemicals better, faster, cheaper, and without fossil fuels from right here in Houston, Texas. Whether you care about the climate, local competitiveness, or just plain old profits, we have good news: it's working," the company states in its news release.
"Our first Bioforge has been operating for a year and Solugen is running a nearly nine figure business with high margins selling commodity and specialty chemicals," the statement continues. "We have established ourselves with top tier customers for our existing solutions and fortune 100 technology partners to build a robust pipeline of future molecules that will help us achieve our goal of 10 mil tons of CO2 removed from the atmosphere."
According to the company, this latest raise has increased Solugen's valuation to over $2 billion. The round was led by investors Kennivik, Lowercarbon Capital, and Refactor Capital.
Houston health tech company wins funding from national organization
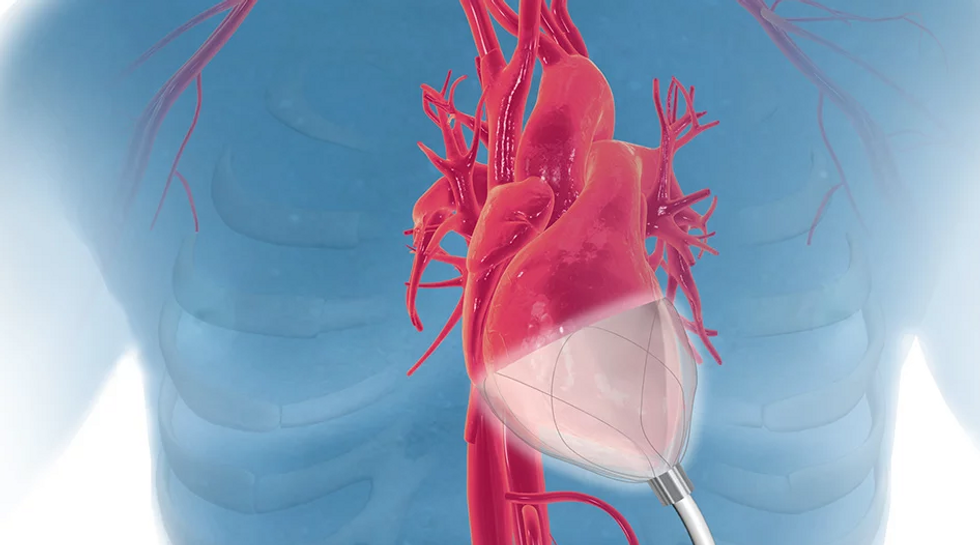
CorInnova has won a prestigious award. Photo via corinnova.com
Houston-based CorInnova was named one of five awardees from the National Capital Consortium for Pediatric Device Innovation's “Make Your Medical Device Pitch for Kids!” competition. Each honoree received a share of $150,000 in grant funding from the U.S. Food and Drug Administration. The awards ranged from $20,000 to $50,000 to support the advancement of pediatric medical devices.
CorInnova has designed a minimally invasive biventricular non-blood contacting cardiac assist device to treat heart failure.
The 2022 competition was moderated by California-based MedTech Innovator. The other four pediatric device innovation awardees included:
- Innovation Lab, from La Palma, California, created a mechanical elbow brace stabilizes tremors for pediatric ataxic cerebral palsy to improve the performance of Activities of Daily Living.
- Prapela, based in Biddeford, Maine, created the first innovation to improve the treatment of apnea of prematurity in over twenty years.
- Tympanogen, from Richmond, Virginia, replaces surgical eardrum repair with a nonsurgical clinic procedure
- Xpan, based in Concord, Ontario, has created a universal trocar enables safest and most dynamic access and effortless upsizing in conventional/mini/robotic procedures.
"We are delighted to recognize these five innovations with critical NCC-PDI funding that will support their journey to commercialization. Improving pediatric healthcare is not possible without forward-thinking companies that seek to address the most dire unmet needs in children’s health,” says Kolaleh Eskandanian, vice president and chief innovation officer at Children’s National Hospital and principal investigator of NCC-PDI, in a news release. "We know all too well how challenging it is to bring pediatric medical devices to market, which is why we have created this rich ecosystem to identify promising medical device technologies and incentivize investment. We congratulate this year’s winning innovators and applaud their efforts to help bridge these important care gaps that are impacting children.”
Houston real estate tech scores funding from Amazon entity

DOSS is a real estate tech company. Photo via Getty Images
Houston-based DOSS, which was chosen this summer for the inaugural Black Founders Build with Alexa cohort, has received funding from the Amazon Alexa Fund. The startups in the program were selected based on their ability to innovate with Alexa and build the next generation of voice, artificial intelligence, and ambient experiences technology.
DOSS is a digital brokerage that uses tech to make homeownership more affordable, and the company has developed a technology where customers are able to ask for real-estate advice and tips, search for home listings, get neighborhood information, and recent sales data, according to a news release from the company. They will also eventually be able to request to be connected with home service providers that serve their respective area.
CEO Bobby Bryant and COO Chris Norton founded DOSS in 2016. Last year, the company participated in the Google for Startups Black Founders program, receiving $100,000 from the fund.
TMC Innovation's Biodesign program applications open
Applications are open through the end of the year. Photo via tmc.edu
Applications are now open for the 2023 TMCi Biodesign program at the Texas Medical Center's Innovation Factory. TMC is looking for candidates with relevant backgrounds for starting a digital health or medical device company, such as: engineering, medicine, hospital administration, R&D (prototyping), software development, finance, legal, regulatory, reimbursement, or technical. Candidates must have at least 1 to 2 years of industry work experience or have prior startup history. It is preferred that applicants have earned an advanced degree.
The position is an in-person, full-time requirement that will begin August 2023 and will span nine months with an opportunity to extend for up to two months.
Applications close on December 31. Candidates will undergo a series of interviews in January and then will be extended offers in the spring.
HX Venture Fund calls for startups to meet visiting VC

Calling all Houston tech startups. Image via Screenshot
HX Venture Fund, a fund-of-funds that encourages and enables non Houston-based VCs to tap into the local innovation ecosystem, is hosting Creighton Hicks, partner at Austin-based LiveOak Venture Partners, later this month.
The organization is looking for Houston startups that are building a tech or tech-enabled services company raising a seed to series B round now or in the next six months. Startups have until November 18 to submit their interest via an online form.