With the Texas Medical Center at the heart of Houston, health advancement opportunities are endless. Medical breakthroughs are happening across town, but as technology advances, the industry is seeing more and more startups popping up to take new tech tools and applying them to traditional medical devices and procedures.
These five Houston startups are developing the future of the industry — one device at a time.
Portable Therapeutix

For years, Squid Compression has helped ease the pain of patients in doctor's offices. Now, anyone can get the treatment on the go. Photo via squidgo.com
The country is currently in an opiod crisis, and one solution is making pain-relieving devices more accessible to patients. That's what Houston-based startup Portable Therapeutix LLC is trying to do with its drug-free solution to pain called Squid Go. The portable device is designed to ease the pain and swelling of sore joints and muscles using cold therapy and compression therapy. It's a follow-up to the company's Squid Compression, a pain management device launched in 2013 for patients at rehabilitation centers, hospitals, doctor's offices, and the like.
To reap the benefits of Squid Go, a consumer uses the device for just 15 minutes. Squid Go — which combines a cold gel pack with proprietary compression technology — features special air pockets that inflate and deflate, gently massaging the body part needing treatment. That massaging boosts circulation and reduces swelling.
"Increased circulation brings more nutrient- and oxygen-rich blood to the area, promoting recovery," says Sam Stolbun, co-founder of Portable Therapeutix. "Meanwhile, [the] gentle compression also drives the pain-relieving cold from the gel pack deeper into the tissues to alleviate soreness and discomfort." Read more about Squid Go here.
Zibrio

Balancing is important throughout your life, and Zibrio has the tools and tips for you to use to stay centered. Pexels
From NASA to your bathroom floor — Katharine Forth has found a new way to track balance. With her company, Zibrio, people can have the everyday ability to figure out how balanced they are.
"The machines typically used for balance measurement can be as large as a telephone booth, so we invented a new way to measure postural control using a much smaller mechanism that fit inside a moon boot," Forth says.
Zibrio is a health company that aims to be the gold standard of measuring balance. The Zibrio scale calculates users' weight like a typical scale and rates their balance on scale of 1 to 10.
The scale gathers data from your weight, your postural control, your muscles and other factors to calculate the rating. Andrea Case-Rogers, chief experience officer at Zibrio, describes a perfect rating of 10 as elusive for most, or "Simone Biles on a good day." Read more about Zibrio here.
Saranas Inc.

Saranas Inc. is testing its technology that can detect and track internal bleeding complications. Getty Images
A Houston-based medical device startup is on a twofold mission to reduce healthcare costs and improve the safety of complex medical procedures involving blood vessels. Saranas Inc. currently in clinical trials for its Early Bird Bleed Monitoring System, which is designed to detect and track bleeding complications related to endovascular procedures. If all goes well, the U.S. Food and Drug Administration will approve Early Bird in 2019, Syed says. Then, the device would be made widely available to medical facilities across the country.
"What attracted me to Saranas is that our solution has the potential to meaningfully reduce serious bleeding complications that worsen clinical outcomes and drive up healthcare costs," says Zaffer Syed, president and CEO of Saranas. "In addition, our device may support access of important minimally invasive cardiac procedures by allowing them to be performed more safely."
Dr. Mehdi Razavi, a cardiologist with the Texas Heart Institute at Houston's Texas Medical Center, invented the device. It's being tested by the institute and other medical facilities in the U.S. As many as 100 patients will participate in the clinical trial, which is expected to last several months. Read more about Saranas and the Early Bird here.
EllieGrid
Courtesy of EllieGrid
Talking the right vitamins and medications at the right times shouldn't seem like more trouble than it's worth. Houston-based EllieGrid is a smart pill box that is easy to organize and stylish to use on the go. Plus, with a growing volume of users, the company is able to use its customer data to track medication compliance.
"What's really neat about EllieGrid is that we are starting to learn users' habits as days go by, so that we can trigger alarms at optimal times," says co-founder and CEO Abe Matamoros at The Cannon's female entrepreneurs pitch night.
For co-founder, Regina Vatterott, the company is about reinventing traditional medical devices that can be clunky or inefficient for daily use.
"We want to do more and more with medical devices because we think that people are always people before they are patients," Vatterott says. Read more about EllieGrid here — as well as more about the founder here.
Intelligent Implants
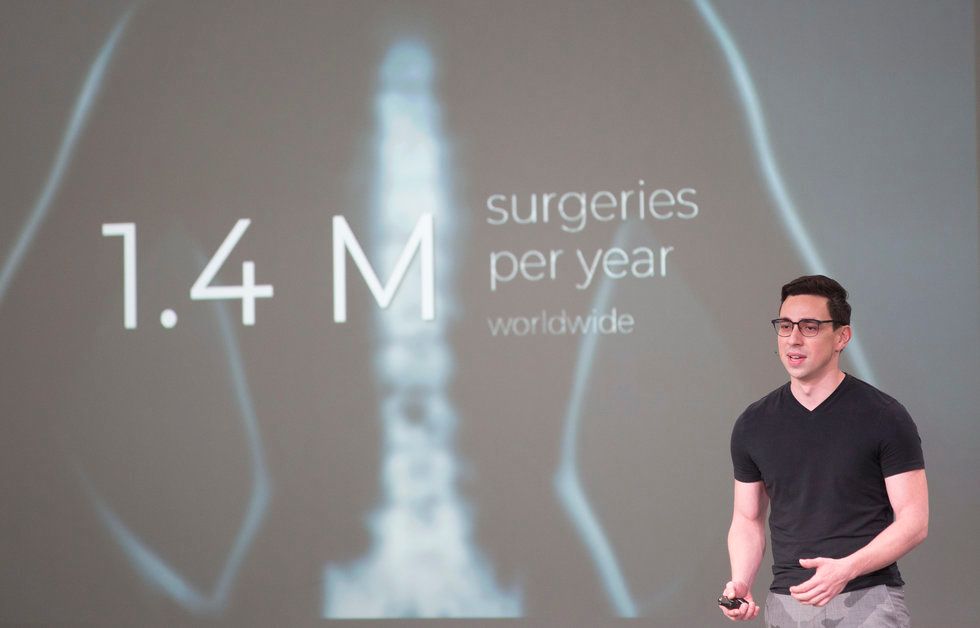
Intelligent Implant's co-founder, Juan Pardo, told the crowd at Demo Day that his company's device allows for 50 percent faster bone growth in patients. Photo by Cody Duty/TMC
Chronic lower back pain can result in a need for spinal fusion surgery — and 40 percent of those surgeries fail, says Juan Pardo, co-founder of Intelligent Implants, which has an office in Houston. Pardo and his team have come up with an implant that tracks post-op healing and introduces electronic stimulation wirelessly.
The device is the same size and shape as the spacer that surgeons currently use, but contains a technology that can deliver electronic stimulation therapy and monitor progress without needing batteries. The doctor is able to adjust treatment remotely, and the device can heal the patient 50 percent faster than the standard care.
Intelligent Implants was announced as the first in-residence company at the Center for Device Innovation by Johnson and Johnson and also launched its large animal studies. The company has a goal to raise $1.6 million, and has already secured $900,000 — $250,000 of which came from the new TMC Venture Fund. Read more about Intelligent Implants and the other Texas companies in its TMC cohort here.




