A Houston medical device company that's tapping into robotics technology for the operating room has just announced a major chunk of fresh funding.
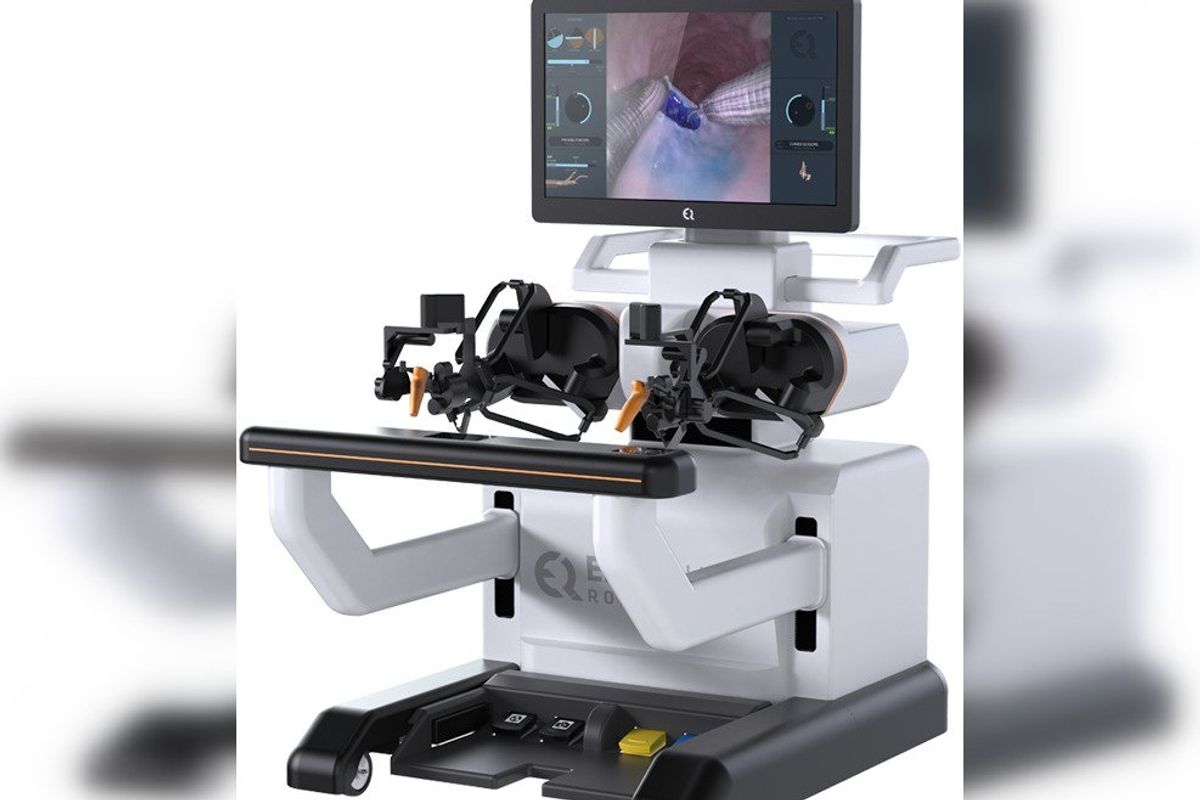
EndoQuest Robotics Inc. announced that it has closed a $42 million series C to advance its robot technology that's targeting endoluminal and gastrointestinal minimally invasive procedures. Returning investors, CE Ventures Limited and McNair Interests, and new investor, Puma Venture Capital, led the round of funding.
"Our investors share our vision of leveraging robotics to redefine the possibilities in minimally invasive procedures," Kurt Azarbarzin, CEO of EndoQuest Robotics, says in a press release. "This financing enables us to continue innovating and refining our technology, ultimately improving patient care and transforming the future of endoluminal interventions."
The funding will go toward continued research and development, regulatory initiatives, commercialization, and other key initiatives. Dr. Vipul Patel, the co-founder and senior venture partner of Puma Venture Capital, is a robotic urologic surgeon and sees potential in EndoQuest's technology.
"I've had the privilege of seeing just about every robotic surgical system either in development or on the market today and can honestly say that EndoQuest's system is a true game changer for both physicians and patients," Patel says in the release.
Founded in 2017, EndoQuest's robotics technology has not yet been cleared by the FDA and is not for commercial sale in the United States.
"The EndoQuest team is trailblazing novel solutions in minimally invasive surgery," Neeraj Agrawal, executive director of Crescent Enterprises, the parent organization to CE Ventures Limited. "We welcome our new partners, and remain fully supportive of the Company and the prospects to transform healthcare with our innovative endoluminal surgical platform."
- Houston medical device company launches is product in the U.S. and hires new exec ›
- Houston medical device startup closes $6M series A ›
- Houston health tech startup acquired by medical device company ›
- Houston medical device startup names new CEO ›
- 5 Houston medical device companies changing the industry ›




