Houston medical device company secures $57.7M to fund journey to FDA approval, commercialization
fresh funding
Houston-born and bred medical device company, Procyrion, has completed its series E with a raise of $57.7 million, including the conversion of $10 million of interim financing.
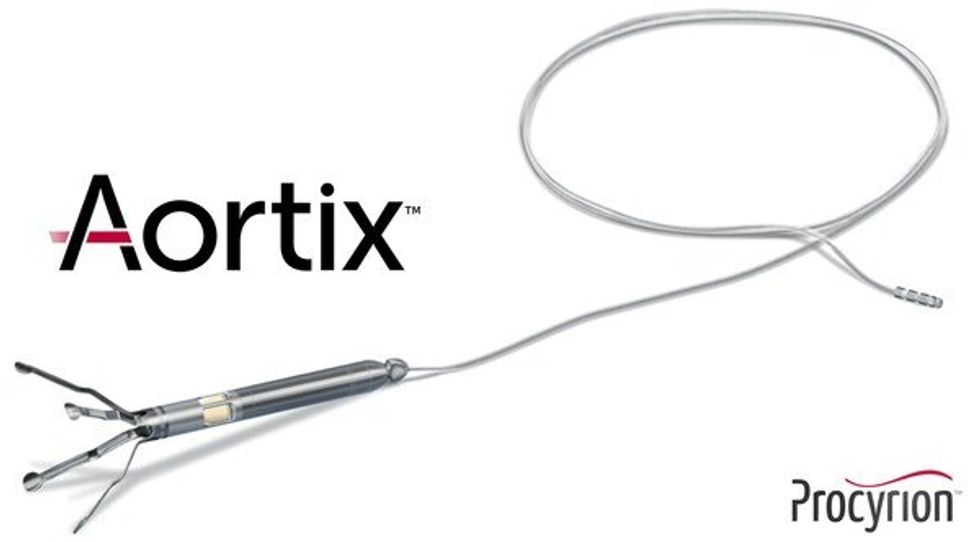
Procyrion is the company behind Aortix, a pump designed to be placed in the descending thoracic aorta of heart failure patients, which has been shown to improve cardiac performance in seriously ill subjects. The money raised will allow the company to proceed with a the DRAIN-HF Study, a pivotal trial that will be used for eventual FDA approval and commercialization.
The Aortix is the brainchild of Houston cardiologist Reynolds Delgado. According to Procyrion’s CSO, Jace Heuring, Delgado, gained some of his experience with devices for the heart working with legendary Texas Heart Institute surgeon O.H. “Bud” Frazier. He filed his first patents related to the Aortix in 2005.
Heuring says that the first prototypes were built in 2011, followed by the final design in 2018. CEO Eric Fain, a California-based MD and with more than 30 years in the medical device industry, joined the company in 2018 ahead of the final design, primed to bring Aortix to the public. He visits the company’s Houston headquarters, across the street from Central Market, on a regular basis.
The device’s pilot study of 18 patients was completed in 2022. Those encouraging results paved the way for the current study, which will include an enrollment of 134 patients. The randomized study will seek to treat patients with acute decompensated heart failure. Half will be treated with standard-of-care therapy, the other half will be catheterized with an Aortix pump. A separate arm of the study will seek to treat end-stage heart failure patients who would otherwise be deemed too sick for either a transplant or an LVAD permanent pump. Fort-five healthcare centers in the United States will participate, including Texas Heart Institute.
“One of the key characteristics is [the patients] are retaining a lot of fluid,” explains Heuring in a video interview. “And when I say a lot, I mean it could be 25 or 30 or 40 pounds of fluid or more. When we put our pump in, one of the main goals is to reduce that fluid load.”
On average, about 11 liters of fluid came off of each patient. Many of those end-stage patients had previously been considered for both a heart and kidney transplant, but after using the Aortix, their kidneys responded so well that they were able to get only the heart transplant.
“These patients really are in dire straits and come into the hospital and today the only proven therapy to help these patients is to administer high doses of intravenous diuretic and some other cardiac drugs and in about 25 percent of patients those therapies are ineffective,” says Fain.
If Aortix gains approval, these sickest of the sick, usually consigned to hospice care, will have hope.
Thanks to the Series E, led by Houston’s Fannin Partners, returning investors, including Bluebird Ventures, the Aortix is inching closer to commercialization. Besides funding the DRAIN-HR study, Procyrion will also use the funds for internal programs to improve product manufacturability. One more step towards meaning advanced heart failure may not always be a death sentence.
Last month, Atul Varadhachary, managing director of Fannin, joined the Houston Innovators Podcast and alluded to Procyrion's raise. The company was born out of Fannin and still resides in the same building as Fannin.

 Apple doubles down on Houston with new production facility, training center Photo courtesy Apple.
Apple doubles down on Houston with new production facility, training center Photo courtesy Apple.