Health tech startup launches Houston study improve stroke patients recovery
now enrolling
A Houston-born company is enrolling patients in a study to test the efficacy of nerve stimulation to improve outcomes for stroke survivors.
Dr. Kirt Gill and Joe Upchurch founded NeuraStasis in 2021 as part of the TMC Biodesign fellowship program.
“The idea for the company manifested during that year because both Joe and I had experiences with stroke survivors in our own lives,” Gill tells InnovationMap. It began for Gill when his former college roommate had a stroke in his twenties.
“It’s a very unpredictable, sudden disease with ramifications not just for my best friend but for everyone in his life. I saw what it did to his family and caregivers and it's one of those things that doesn't have as many solutions for people to continue recovery and to prevent damage and that's an area that I wanted to focus myself on in my career,” Gill explains.
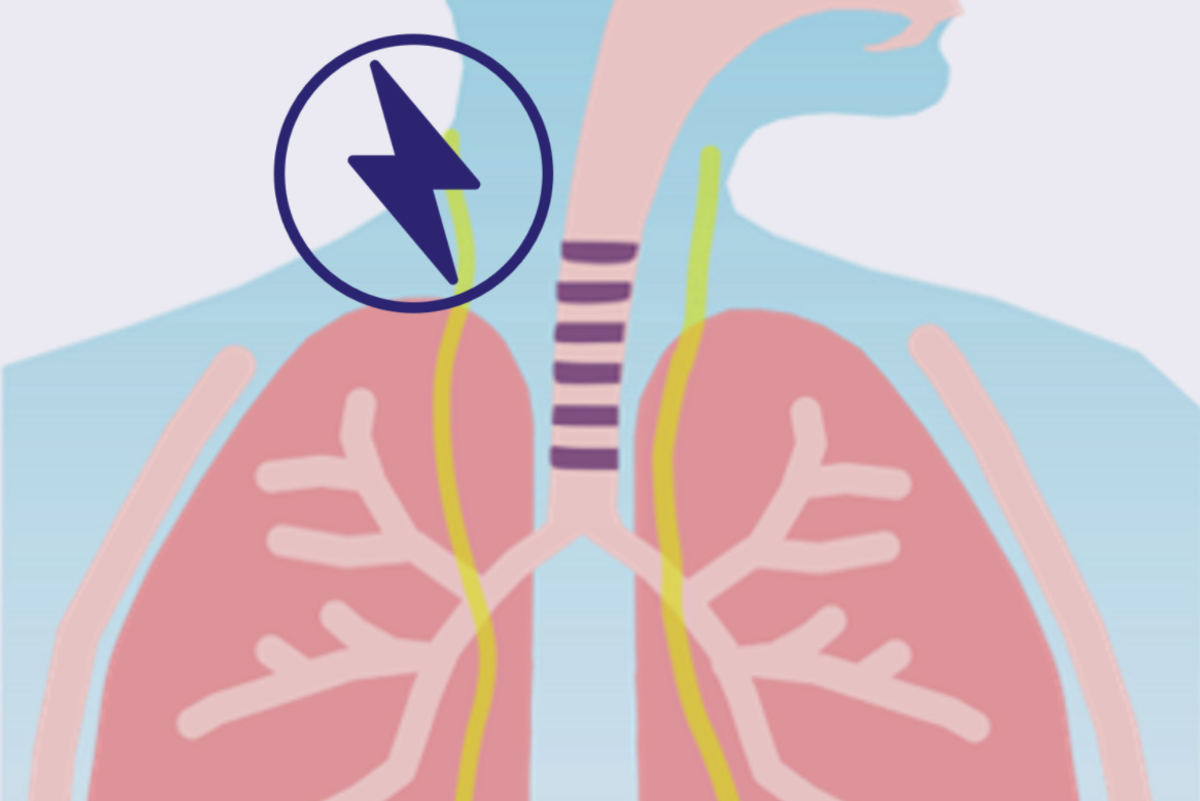
Gill and Upchurch arrived at the trigeminal and vagus nerves as a potential key to helping stroke patients. Gill says that there is a growing amount of academic literature that talks about the efficacy of stimulating those nerves. The co-founders met Dr. Sean Savitz, the director of the UTHealth Institute for Stroke and Cerebrovascular Diseases, during their fellowship. He is now their principal investigator for their clinical feasibility study, located at his facility.
The treatment is targeted for patients who have suffered an ischemic stroke, meaning that it’s caused by a blockage of blood flow to the brain.
“Rehabilitation after a stroke is intended to help the brain develop new networks to compensate for permanently damaged areas,” Gill says. “But the recovery process typically slows to essentially a standstill or plateau by three to six months after that stroke. The result is that the majority of stroke survivors, around 7.6 million in the US alone, live with a form of disability that prevents complete independence afterwards.”
NeuraStasis’ technology is intended to help patients who are past that window. They accomplish that with a non-invasive brain-stimulation device that targets the trigeminal and vagus nerves.
“Think of it kind of like a wearable headset that enables stimulation to be delivered, paired to survivors going through rehabilitation action. So the goal here is to help reinforce and rewire networks as they're performing specific tasks that they're looking to improve upon,” Gill explains.
The study, which hopes to enroll around 25 subjects, is intended to help people with residual arm and hand deficits six months or more after their ischemic stroke. The patients enrolled will receive nerve stimulation three times a week for six weeks. It’s in this window that Gill says he hopes to see meaningful improvement in patients’ upper extremity deficits.
Though NeuraStasis currently boasts just its two co-founders as full-time employees, the company is seeing healthy growth. It was selected for a $1.1 million award from the National Institutes of Health through its Blueprint MedTech program. The award was funded by the National Institute of Neurological Disorders and Stroke. The funding furthers NeuraStasis’ work for two years, and supports product development for work on acute stroke and for another product that will aid in emergency situations.
Gill says that he believes “Houston has been tailor-made for medical healthcare-focused innovation.”
NeuraStasis, he continues, has benefited greatly from its advisors and mentors from throughout the TMC, as well as the engineering talent from Rice, University of Houston and Texas A&M. And the entrepreneur says that he hopes that Houston will benefit as much from NeuraStasis’ technology as the company has from its hometown.
“I know that there are people within the community that could benefit from our device,” he says.

 Over 1,000 companies applied to participate in the 2023 MedTech Innovator Accelerator, 200 pitched in person, and 61 startups were selected. Graphic via
Over 1,000 companies applied to participate in the 2023 MedTech Innovator Accelerator, 200 pitched in person, and 61 startups were selected. Graphic via