These 7 Houston health tech companies are providing COVID-19 solutions
startups to the rescue
It's all hands on deck in Houston in the battle against coronavirus — and local biotech startups have risen to the occasion.
From mental health solutions and online portals to virtual medicine and new treatments, these Houston companies have recently launched or pivoted to new options in health care.
Mental Health Match

Ryan Schwartz is offering free counseling to Texans. Photo courtesy of Mental Health Match
Mental Health Match, a Houston-based startup that uses tech to easily connect people to mental health professionals, has announced the opportunity for 100 Texas residents to get their first appointment free and remotely.
The company cites data that shows:
- A 78 percent increase in Texans who are concerned about their marriage
- A 71 percent increase in Texans concerned with their parenting or their children
- A 35 percent spike in Texans feeling panicked
"You might be practicing social distancing, but you are not alone. It is easier to make it through this together if you can get support and guidance from a skilled professional. That's why we're working with therapists across Texas to provide a free session to individuals who need it most," says Ryan Schwartz, founder of Mental Health Match, in a news release.
Texans can apply for the free sessions online on a first-come, first-served basis.
Medical Informatics Corp.
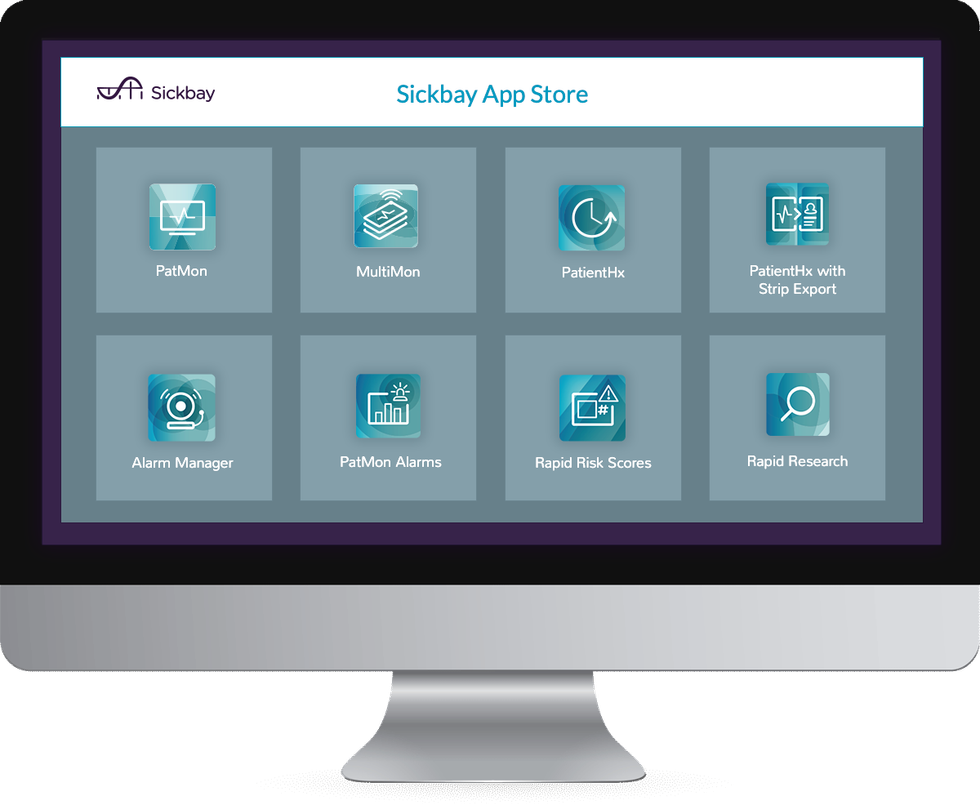
Medical Informatics Corp.'s Sickbay platform can monitor patients from afar. Photo via michealthcare.com
Houston-based Medical Informatics has created a virtual ICU program, called Sickbay, and the tech tool is being used to remotely monitor patients in Houston Methodist. The program works around the clock from a control hub to use artificial intelligence and algorithms to monitor patients.
The company, which recently moved into its office in TMCx+, announced major growth in January, just ahead of the coronavirus outbreak.
"We designed our Sickbay platform to give lost data back to doctors, nurses and other members of the care team so they could save more lives," says Vincent Gagne, vice president of product for MIC, in a news release. "In fact, our apps are built in collaboration with our clients, such as Texas Children's Hospital and Houston Methodist. Having these facilities blocks away from our headquarters accelerates that collaboration and development."
MolecularMatch

MolecularMatch is bringing together COVID-19 information and trials. Photo via molecularmatch.com
MolecularMatch, a Houston startup focused on clinical informatics, has launched a free portal that accumulates research and clinical trials for COVID-19. The company is a tenant of TMCx+ and a portfolio company of Houston-based venture capital group GOOSE.
"The number of therapeutic cures and vaccines being tested are growing at an astounding rate," says Eric Pulaski, CEO at MolecularMatch, in a news release. "Our tools make it easier for clinicians and patients to find the help they need. Hopefully, we can help save lives by shortening the time it takes to get more patients into clinical trials and by speeding up research to find cures and vaccines."
The product uses the company's artificial intelligence-backed curation platform and is updated every two to three days.
Luminare
Luminare Inc. pivoted to quickly create an online COVID-19 screening tool, and local governments have tapped into the resource. Andriy Onufriyenko/Getty Images
Founded in 2014, Houston-based health care software startup Luminare Inc. seeks to prevent sepsis, a life-threatening reaction to a host of infections that causes about one-third of U.S. hospital deaths. Recently, though, Luminare pivoted to address another health concern — the threat of the novel coronavirus.
After the novel coronavirus surfaced, Luminare retooled its sepsis-detection platform to create a free online self-assessment test for people who suspect they've contracted the virus. The test, available at CheckForCorona.com, helps someone figure out whether they should seek a coronavirus test.
An online screening typically takes less than two minutes. The confidential, secure assessment complies with guidelines from the U.S. Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO). Based on your assessment results, you might be directed to contact your local health department or, in the worst-case scenario, call 911. Click here to read more.
Manatee
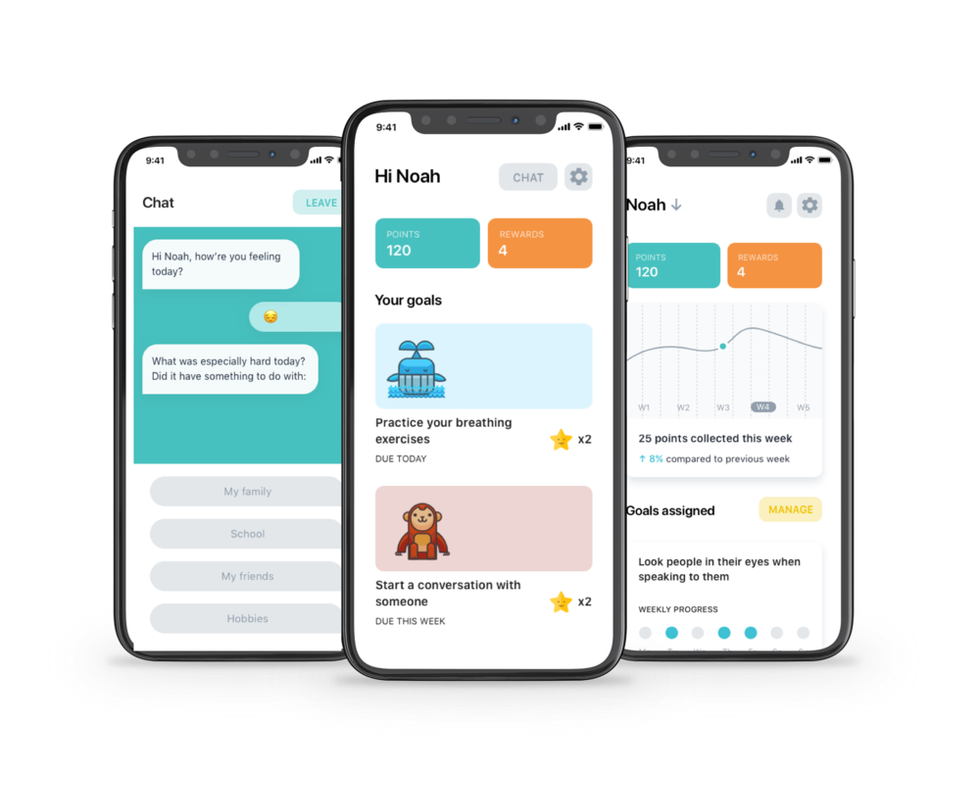
Manatee users can sign up for three months free. Photo via getmanatee.com
Denver-based Manatee was just announced to be selected for the 2020 TMCx cohort, and — while programming is beginning virtually — the startup will be enroute to Houston as soon as it's safe. Manatee focuses on providing connected, everyday therapy for kids.
In light of the effects of COVID-19 on both parents and children, Manatee has allowed users to register for three months free. Individuals can apply online.
Moleculin Biotech Inc.

Houston-based Moleculin, which works on oncology treatment, has filed a patent for its treatment to battle the coronavirus. Getty Images
Houston-based Moleculin Biotech Inc., a clinical stage pharmaceutical company that typically focuses on cancer treatment, announced that it has filed for a new patent for its use of one of its products to be used against the coronavirus and other potential viruses.
This patent application is for Moleculin's WP1122, and the company has entered into a partnership with a major Texas university to advance its research.
"We've actually been working on the antiviral potential of WP1122 for some time now," says Walter Klemp, Moleculin's chairman and CEO, in a news release, "but the rise of COVID-19 has obviously placed a new sense of urgency on what we are doing. We hope to be generating animal data on WP1122's antiviral potential in the near term."
Pulmotech Inc.
Pulmotect, a clinical-stage biotechnology company based in Houston, is testing a drug that could be useful in mitigating the threats of the coronavirus, which is currently been recognized as a global health emergency. Getty Images
Experiments conducted by clinical-stage, Houston-based biotechnology company Pulmotect Inc. show its PUL-042 inhaled drug has proven effective in protecting mice against two types of coronavirus: severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). Researchers performed those tests at the University of Texas Medical Branch at Galveston.
In the Galveston experiments, a single inhaled dose of PUL-042 protected lab mice from the SARS virus, and it greatly reduced the amount of virus in their lungs after the mice became infected with SARS or MERS.
"With the risks of virulent coronaviruses and other threats increasing, as shown by the recent outbreak in Wuhan that has already spread from China to other countries including the United States, Pulmotect is optimistic that its immune-stimulating technology could be useful in mitigating the threats of current and emerging pathogens and protecting vulnerable populations," says CEO Dr. Colin Broom in a news release. Click here to read more.
