Houston engineers develop breakthrough device to advance spinal cord treatment
future of health
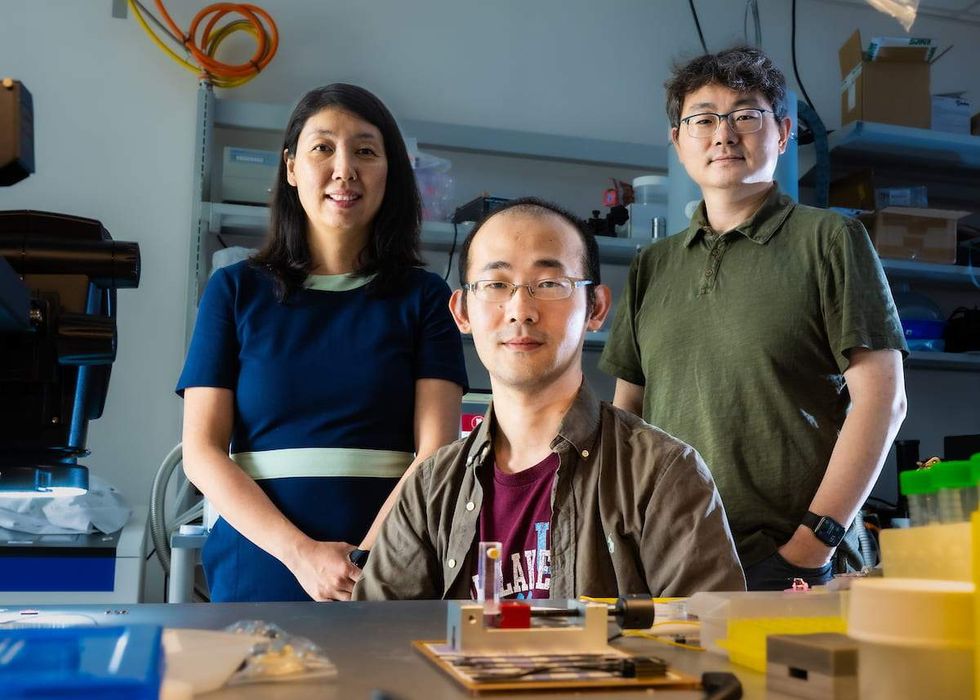
A team of Rice University engineers has developed an implantable probe over a hundred times smaller than the width of a hair that aims to help develop better treatments for spinal cord disease and injury.
Detailed in a recent study published in Cell Reports, the probe or sensor, known as spinalNET, is used to explore how neurons in the spinal cord process sensation and control movement, according to a statement from Rice. The research was supported by the National Institutes of Health, Rice, the California-based Salk Institute for Biological Studies, and the philanthropic Mary K. Chapman Foundation based in Oklahoma.
The soft and flexible sensor was used to record neuronal activity in freely moving mice with high resolution for multiple days. Historically, tracking this level of activity has been difficult for researchers because the spinal cord and its neurons move so much during normal activity, according to the team.
“We developed a tiny sensor, spinalNET, that records the electrical activity of spinal neurons as the subject performs normal activity without any restraint,” Yu Wu, a research scientist at Rice and lead author of the study said in a statement. “Being able to extract such knowledge is a first but important step to develop cures for millions of people suffering from spinal cord diseases.”
The team says that before now the spinal cord has been considered a "black box." But the device has already helped the team uncover new findings about the body's rhythmic motor patterns, which drive walking, breathing and chewing.
"Some (spinal neurons) are strongly correlated with leg movement, but surprisingly, a lot of neurons have no obvious correlation with movement,” Wu said in the statement. “This indicates that the spinal circuit controlling rhythmic movement is more complicated than we thought.”
The team said they hope to explore these findings further and aim to use the technology for additional medical purposes.
“In addition to scientific insight, we believe that as the technology evolves, it has great potential as a medical device for people with spinal cord neurological disorders and injury,” Lan Luan, an associate professor of electrical and computer engineering at Rice and a corresponding author on the study, added in the statement.
Rice researchers have developed several implantable, minimally invasive devices to address health and mental health issues.
In the spring, the university announced that the United States Department of Defense had awarded a four-year, $7.8 million grant to the Texas Heart Institute and a Rice team led by co-investigator Yaxin Wang to continue to break ground on a novel left ventricular assist device (LVAD) that could be an alternative to current devices that prevent heart transplantation.
That same month, the university shared news that Professor Jacob Robinson had published findings on minimally invasive bioelectronics for treating psychiatric conditions. The 9-millimeter device can deliver precise and programmable stimulation to the brain to help treat depression, obsessive-compulsive disorder and post-traumatic stress disorder.- Houston innovation hub restructures, pulls in more Rice resources ›
- Rice University's edtech company receives $90M to lead NSF research hub ›
- Houston organization introduces inaugural cancer-fighting cohort of data sciences, experts ›
- These elite Houston researchers were named among the most-cited in their fields ›

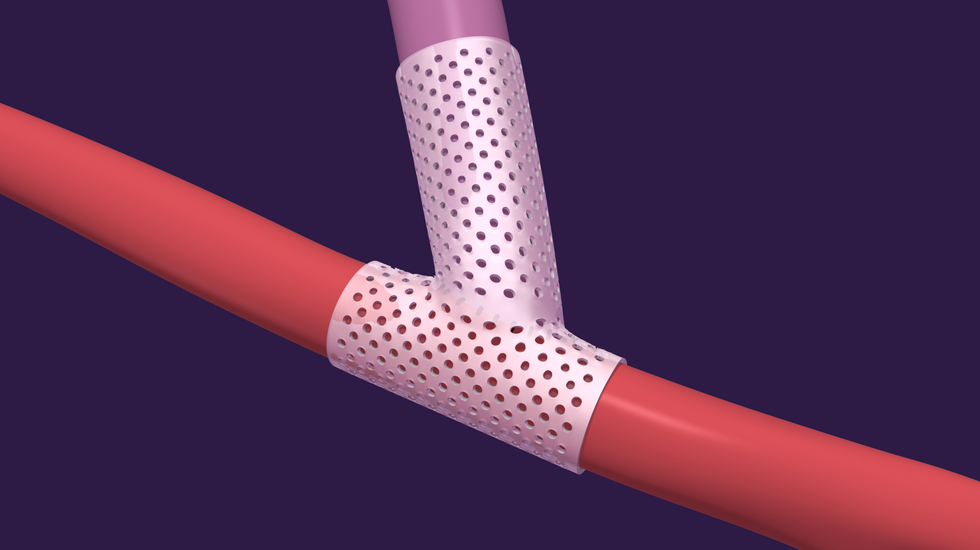 VenoStent's novel therapeutic medical device is a bioabsorbable wrap. Image courtesy of VenoStent
VenoStent's novel therapeutic medical device is a bioabsorbable wrap. Image courtesy of VenoStent Tim Boire is the CEO of VenoStent. Photo via LinkedIn
Tim Boire is the CEO of VenoStent. Photo via LinkedIn


