Houston data scientist joins medical device startup amid AI evolution in the sector
HOUSTON INNOVATORS PODCAST EPISODE 241
When most people hear about Houston startup Starling Medical, they might think about how much potential the medical device company has in the field of urinalysis diagnostics. But that's not quite where Angela Wilkins's head went.
Wilkins explains on the Houston Innovators Podcast that when she met the company's co-founders, Hannah McKenney and Drew Hendricks, she recognized them as very promising startup leaders taking action on a real health care problem. Starling's device can collect urine and run diagnostics right from a patient's toilet.
"It was one of those things where I just thought, 'They're going to get a bunch of data soon,'" Wilkins says. "The opportunity is just there, and I was really excited to come on and build their AI platform and the way they are going to look at data."
For about a year, Wilkins supported the startup as an adviser. Now, she's working more hands on as chief data officer as the company grows.
Wilkins, who serves as a mentor and adviser for several startups, has a 20-year career in Houston across all sides of the innovation equation, working first at Baylor College of Medicine before co-founding Mercury Data Science — now OmniScience. Most recently she served as executive director of the Ken Kennedy Institute at Rice University.
This variety in her resume makes her super connective — a benefit to all the startups she works with, she explains. The decision to transition to a startup team means she gets to work hands on in building a technology — while bringing in her experience from other institutions.
"I think I've really learned how to partner with those institutions," she says on the show. "I've really learned how to make those bridges, and that's a big challenge that startups face."
"When we talk about the Houston innovation ecosystem, it's something we should be doing better at because we have so many startups and so many places that would like to use better technology to solve problems," she continues.
Wilkins has data and artificial intelligence on the mind in everything she does, and she even serves on a committee at the state level to learn and provide feedback on how Texas should be regulating AI.
"At the end of the day, the mission is to put together a report and strategy on how we think Texas should think about AI," she explains. "It's beyond just using an algorithm, they need infrastructure."
Colorado is the first state to pass legislation surrounding AI, and Wilkins says all eyes are on how execution of that new law will go.
"We should have technology that can be double checked to make sure we're applying it in a way that's fair across all demographics. It's obvious that we should do that — it's just very hard," she says.

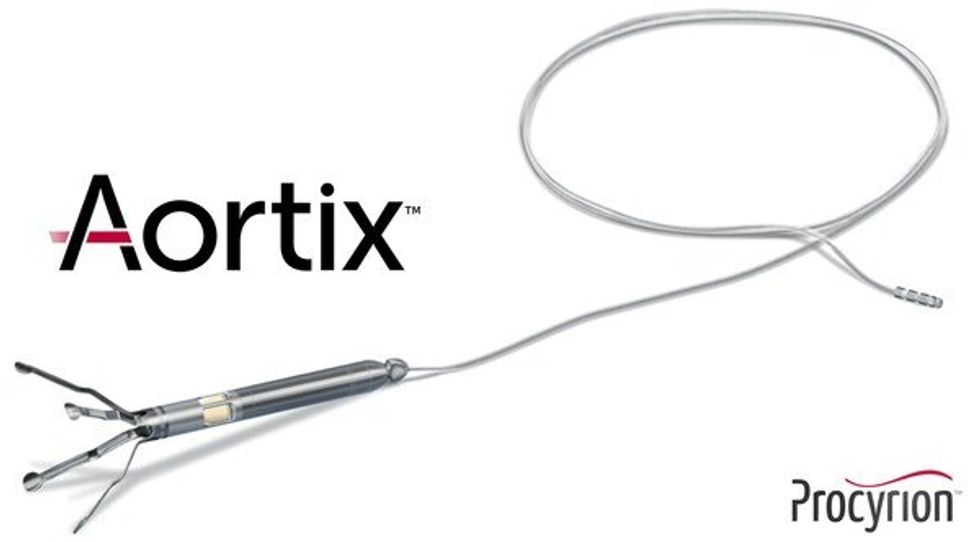 Aortix is a pump designed to be placed in the descending thoracic aorta of heart failure patients. Photo via Procyrion
Aortix is a pump designed to be placed in the descending thoracic aorta of heart failure patients. Photo via Procyrion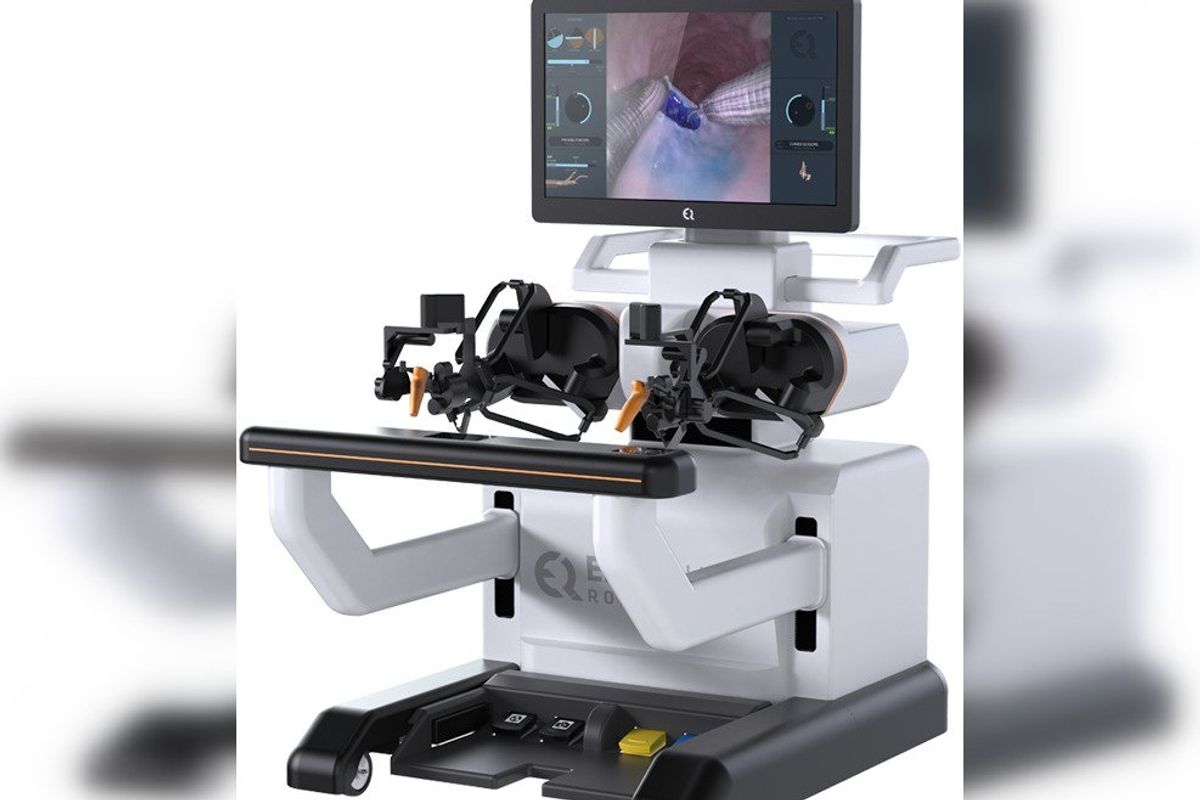 EndoQuest Robotics is targeting endoluminal and gastrointestinal minimally invasive procedures. Image via endoquestrobotics.com
EndoQuest Robotics is targeting endoluminal and gastrointestinal minimally invasive procedures. Image via endoquestrobotics.com