Axiom Space-tested cancer drug advances to clinical trials
mission critical
A cancer-fighting drug tested aboard several Axiom Space missions is moving forward to clinical trials.
Rebecsinib, which targets a cancer cloning and immune evasion gene, ADAR1, has received FDA approval to enter clinical trials under active Investigational New Drug (IND) status, according to a news release. The drug was tested aboard Axiom Mission 2 (Ax-2) and Axiom Mission 3 (Ax-3). It was developed by Aspera Biomedicine, led by Dr. Catriona Jamieson, director of the UC San Diego Sanford Stem Cell Institute (SSCI).
The San Diego-based Aspera team and Houston-based Axiom partnered to allow Rebecsinib to be tested in microgravity. Tumors have been shown to grow more rapidly in microgravity and even mimic how aggressive cancers can develop in patients.
“In terms of tumor growth, we see a doubling in growth of these little mini-tumors in just 10 days,” Jamieson explained in the release.
Rebecsinib took part in the patient-derived tumor organoid testing aboard the International Space Station. Similar testing is planned to continue on Axiom Station, the company's commercial space station that's currently under development.
Additionally, the drug will be tested aboard Ax-4 under its active IND status, which was targeted to launch June 25.
“We anticipate that this monumental mission will inform the expanded development of the first ADAR1 inhibitory cancer stem cell targeting drug for a broad array of cancers," Jamieson added.
According to Axiom, the milestone represents the potential for commercial space collaborations.
“We’re proud to work with Aspera Biomedicines and the UC San Diego Sanford Stem Cell Institute, as together we have achieved a historic milestone, and we’re even more excited for what’s to come,” Tejpaul Bhatia, the new CEO of Axiom Space, said in the release. “This is how we crack the code of the space economy – uniting public and private partners to turn microgravity into a launchpad for breakthroughs.”
- Houston company prepares for takeoff of first commercial space launch ›
- Houston space tech co. revises timeline for commercial space station development with NASA partnership ›
- Texas Space Commission doles out $5.8 million to Houston companies ›
- Houston space tech startups share latest updates on lunar missions and more ›
- Axiom Space wins NASA contract for fifth private mission to ISS - InnovationMap ›

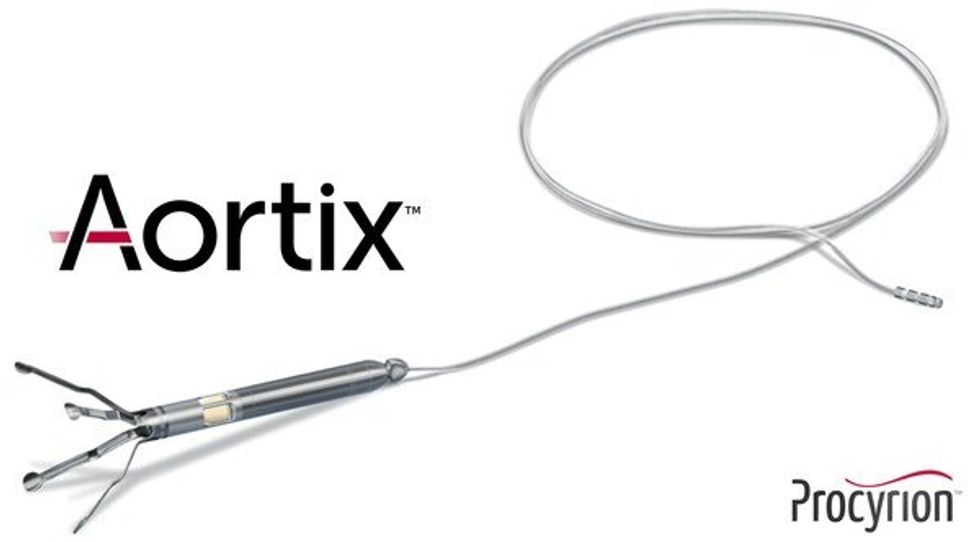 Aortix is a pump designed to be placed in the descending thoracic aorta of heart failure patients. Photo via Procyrion
Aortix is a pump designed to be placed in the descending thoracic aorta of heart failure patients. Photo via Procyrion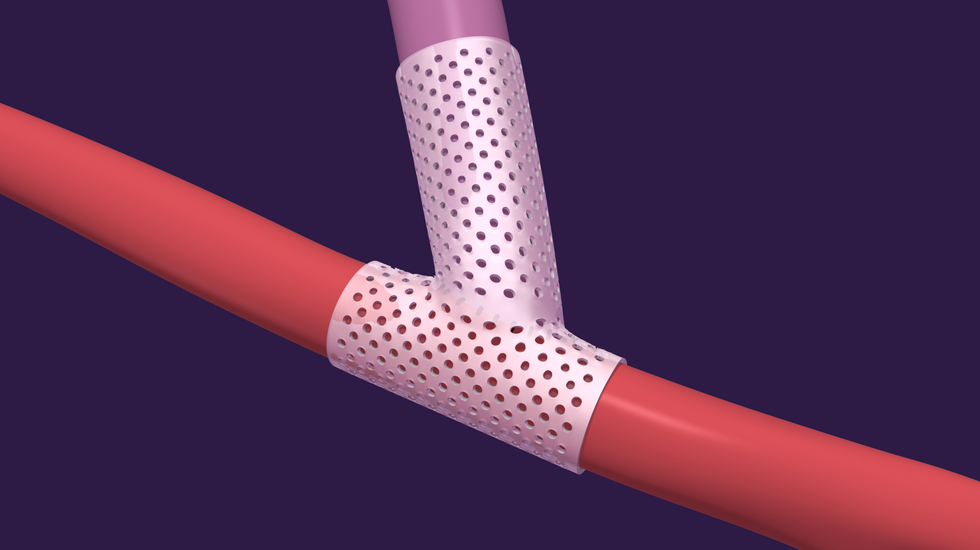 VenoStent's novel therapeutic medical device is a bioabsorbable wrap. Image courtesy of VenoStent
VenoStent's novel therapeutic medical device is a bioabsorbable wrap. Image courtesy of VenoStent Tim Boire is the CEO of VenoStent. Photo via LinkedIn
Tim Boire is the CEO of VenoStent. Photo via LinkedIn