Robotic device created at the University of Houston helps stroke patients to rehabilitate
next-gen recovery
Almost 800,000 people in the United States suffer from a stroke annually — and the affliction affects each patient differently. One University of Houston researcher has created a device that greatly improves the lives of patients whose stroke affected motor skills.
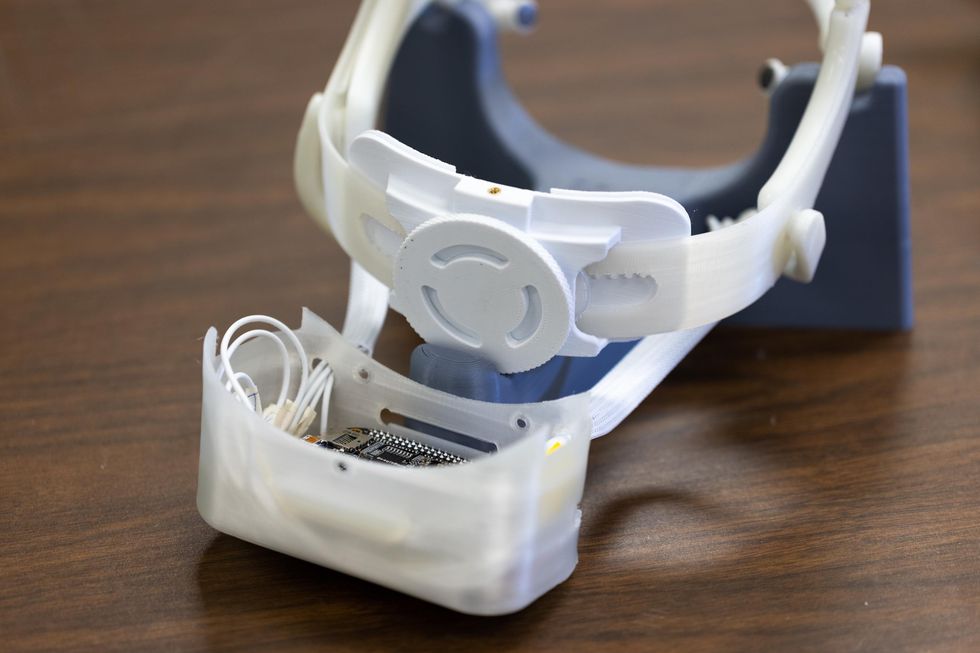
UH engineering professor Jose Luis Contreras-Vidal developed a next-generation robotic arm that can be controlled by the user's brainwaves. The portable device uses a brain-computer interface (BCI) developed by Contreras-Vidal. Stroke patient Oswald Reedus, 66, is the first person to use a device of this kind.
Reedus lost the use of his left arm following a stroke that also caused aphasia, or difficulty speaking. While he's been able to recover his ability to speak clearly, the new exoskeleton will help rehabilitate his arm.
When strapped into the noninvasive device, the user's brain activity is translated into motor commands to power upper-limb robotics. As patients like Reedus use the device, more data is collected to improve the experience.
“If I can pass along anything to help a stroke person’s life, I will do it. For me it’s my purpose in life now,” says Reedus in a news release from UH. His mother and younger brother both died of strokes, and Reedus is set on helping the device that can help other stroke patients recover.
Contreras-Vidal, a Hugh Roy and Lillie Cranz Cullen distinguished professor, has led his device from ideation to in-home use, like with Reedus, as well as clinical trials at TIRR Memorial Hermann. The project is funded in part from an $813,999 grant from the National Science Foundation’s newly created Division of Translational Impacts.
"Our project addresses a pressing need for accessible, safe, and effective stroke rehabilitation devices for in-clinic and at-home use for sustainable long-term therapy, a global market size expected to currently be $31 billion," Contreras-Vidal says in the release. "Unfortunately, current devices fail to engage the patients, are hard to match to their needs and capabilities, are costly to use and maintain, or are limited to clinical settings."
Dr. Gerard E. Francisco, chief medical officer and director of the Neuro Recovery Research Center at TIRR Memorial Hermann, is leading the clinical trials for the device. He's also chair and professor in the Department of Physical Medicine and Rehabilitation at McGovern Medical School at UTHealth Houston. He explains that TIRR's partnership with engineering schools such as the Cullen College of Engineering at UH and others around the nation is strategic.
“This is truly exciting because what we know now is there are so many ways we can induce neuroplasticity or how we can boost recovery,” says Francisco in the release. “That collaboration is going to give birth to many of these groundbreaking technologies and innovations we can offer our patients.”